Physics Notes Form 1
Physics Notes Form 1
Physics Notes Form 1
Chapter One
Physics Form One
Introduction to Physics
Science in our lives
Scientists are people trained in science and who practice the knowledge of science.
We require people in industries to work as engineers, technicians, researchers, in hospitals as doctors, nurses and technologists.
Science gives us powerful ideas, instruments and methods which affect us in our daily lives.
Scientific methods
1. A laboratory is a building specifically designed for scientific work and may contain many pieces of apparatus and materials for use.
2. A hypothesis is a scientific fact or statement that has not been proven or experimented.
3. A law or principle is a scientific fact or statement that has been proven and experimented to be true for all conditions.
4. A theorem is a fact or statement that is true and proven but applicable under specific conditions.
What is physics?
Physics is a Greek word meaning nature hence it deals with natural phenomena.
Physics is therefore a science whose objective is the study of components of matter and their mutual interactions.
Physics is also defined as the study of matter and its relation to energy.
A physicist is able to explain bulk properties of matter as well as other phenomena observed.
Branches of physics
1. Mechanics – the study of motion of bodies under the influence of force.
2. Electricity – this deals with the movement of charge from one point to another through a conductor.
3. Magnetism – the study of magnets and magnetic fields and their extensive applications.
4. Thermodynamics / heat – this is the study of the transformation of heat from one form to another.
5. Optics –the study of light as it travels from one media to another.
6. Waves – the study of disturbances which travel through mediums or a vacuum.
7. Particle physics
8. Nuclear physics
9. Plasma physics
Relation of physics to other subjects
Since physics enables us to understand basic components of matter and their mutual interactions it forms the base of natural science.
Biology and chemistry borrow from physics in explaining processes occurring in living things and organisms.
Physics also provides techniques which are applied almost every area of pure and applied science i.e.
meteorology, astronomy etc.
Career opportunities in physics
1 Engineering – civil
- Electrical
- Mechanical
- Agricultural
- Environmental
- Chemical
- Computer2. Meteorology
3. Surveying
4. Geology
5. Astronomy
NOTE: – all science based careers i.e. doctors, nurses, technologists, engineers, pharmacists etc. need physics as a true foundation.
Basic laboratory safety rules
1. Proper dressing must be observed, no loose clothing, hair and closed shoes must be worn.
2. Identify the location of electricity switches, fire-fighting equipment, first aid kit, gas and water supply systems.
3. Keep all windows open whenever working in the laboratory.
4. Follow all instructions carefully and never attempt anything in doubt.
5. No eating or drinking allowed in the laboratory.
6. Ensure that all electrical switches, gas and water taps are turned off when not in use.
7. Keep floors and working surfaces dry. Any spilla ge must be wiped off immediately.
8. All apparatus must be cleaned and returned in the correct location of storage after use.
9. Hands must be washed before leaving the laboratory.
10. Any accidents must be reported to the teacher immediately.
Chapter Two
Measurement
In order to measure we need to know or define the quantity to be measured and the units for measuring it.
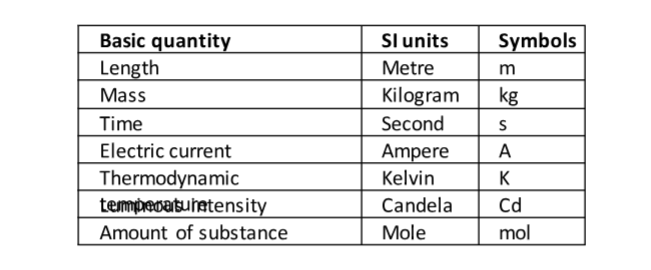
In 1971 a system known as the International System of Units (Systeme’ Internationale) and seven basic units were agreed upon as follows. Other quantities can be obtained from these basic quantities and are referred to as derived quantities.

LengthThis is the measure of distance between two points in space. The SI unit for length is the metre (m).Therefore 1 km = 1000 m
1 Hm = 100 m
1 Dm= 10 m
1 mm = 0.001 m
Length is measured using a metre rule (100 cm), tape measure (100 m, 300 m, 500 m)
Area
This is the measure of the extent of a surface. It is a derived quantity of length. Its SI units are square metres (m2). Other units are cm2, km2, etc.
Formulas are used to determine areas of regular bodies while for irregular bodies an approximation of area is used.
Volume
This is the amount of space occupied by matter. The SI units for volume is cubic metre (m3). Other sub-multiples are cm3, mm3 and l.
Hence 1 m3 = 1,000,000 cm3 and 1l= 1,000 cm3. Volume can be measured using a measuring cylinder, eureka can, pipette, burette, volumetric flask, beaker, etc.
Mass
This is the quantity of matter contained in a substance . Matter is anything that occupies space and has weight. The SI unit for mass is the Kilogram (kg).
Other sub-multiples used are grams (g), milligrams (mg) and tonnes (t). 1 kg = 1,000 g = 1,000,000 mg=100 tonnes. A beam balance is used to measure mass.
Density
This is mass per unit volume of a substance. It is symbolized by rho (ρ) and its SI units are kg/m3.
Density = mass / volume.
Examples
1. A block of glass of mass 187.5 g is 5.0 cm long, 2.0 cm thick and 7.5 cm high. Calculate the density of the glass in kgm -3.
Solution
Density = mass / volume = (187.5 /1000) /(2.0 × 7.5 × 5.0 /1,000,000) = 2,500 kgm-3.
2. The density of concentrated sulphuric acid is 1.8 g/cm 3. Calculate the volume of 3.1 kg of the acid.
Solution
Volume = mass / density = 3,100 / 1.8 = 1,722 cm3 or 0.001722 m3.
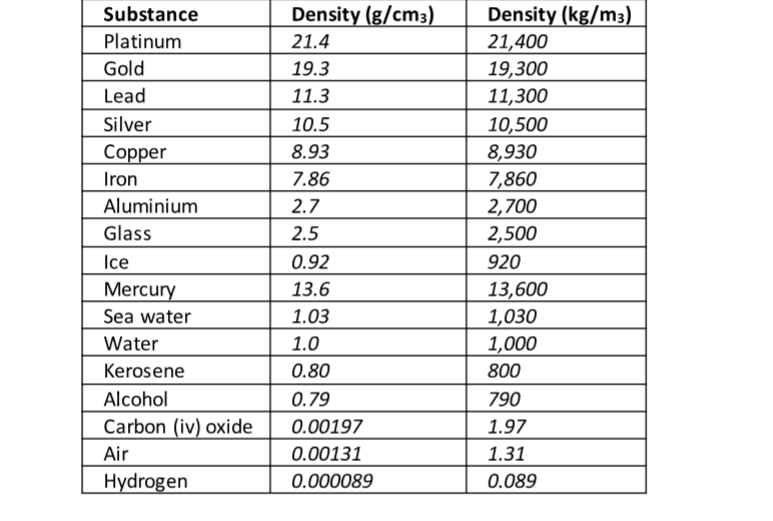
The following is a list of dens ities of some common substances

ExampleThe mass of an empty density bottle is 20 g. Its mass when filled with water is 40.0 g an d 50.0 g when filled with liquid X. Calculate the density of liquid X if the density of water is 1,000 kgm-3.
Solution
Mass of water = 40 – 20 = 20 g = 0.02 kg.
Volume of water = 0.02 / 1,000 = 0.00002 m3. Volume of liquid = volume of bottle
Mass of liquid = 50 – 20 = 30 g = 0.03 kg
Therefore density of liquid = 0.03 / 0.00002 = 1,500 kgm-3
Relative density
This is the density of a substance compared to the density of water.
It is symbolized by (d) and has no units since it’s a ratio.
Relative density (d) = density of substance / density of water. It is measured using a relative density bottle
Example
The relative density of some type of wood is 0.8. Find the density of the wood in kg/m 3.
Solution
Density of substance = d × density of water
Density of subs tance = 0.8 × 1,000 = 800 kgm-3
Densities of mixtures
We use the following formula to calculate densities of mixtures
Density of the mixture = mass of the mixture / volume of the mixture
Example
100 cm3 of fresh water of density 1,000 kgm-3 is mixed with 100 cm3 of sea water of density 1030 kgm-3.
Calculate the density of the mixture.
Solution
Mass = density × volume
Mass of fresh water = 1,000 × 0.0001 = 0.1 kg
Mass of sea water = 1030 × 0.0001 = 0.103 kg
Mass of mixture = 0.1 + 0.103 = 0.203 kg
Volume of mixture = 100 + 100 = 200 cm3 = 0.0002 m3
Therefore density = mass / volume = 0.203 / 0.0002 =1,015 kg/m3.
Time
This is a measure of duration of an event . The SI unit for time is the second (s). Sub- multiples of the second are milliseconds, microseconds, minute, hour, day, week and year.
It is measured using clocks, stop watches, wrist watches, and digital watches.
Accuracy and errors
Accuracy is the closeness of a measurement to the correct value of the quantity being measured.
It is expressed as an error.
An error is therefore the deviation of measurement to the correct value being measured.
The smaller the error the accurate the measurement.
% error = (sensitivity / size measured) × 100.
Chapter Three
Forces.
Force is a push or a pull. Force is therefore that which changes a body’s state of motion or shape.
The SI unit for force is Newton (N). It is a vector quantity. It is represented by the following symbol.
Types of forces1. Gravitational force –this is the force of attraction between two bodies of given masses.
– Earth’s gravitational force is the force which pulls a body towards its center. This pull of gravity is called weight.
2. Force of friction – this is a force which opposes the relative motion of two surfaces in contact with each other. Friction in fluids is known as viscosity.
3. Tension force – this is the pull or compression of a string or spring at both its ends.
4. Upthrust force – this is the upward force acting on an object immersed in a fluid.
5. Cohesive and adhesive forces – cohesive is the force of attraction of molecules of the same kind while adhesive is the force of attraction of molecules of different kinds .
6. Magnetic force – this is a force which causes attraction or repulsion in a magnet.
7. Electrostatic force – this is the force of attraction or repulsion of static charges.
8. Centripetal force – this is a force which constrains a body to move in a circular orbit or path.
9. Surface tension – this is the force which causes the surface of a liquid to behave like a stretched skin. This force is cohesive.
Factors affecting surface tension
a) Impurities – they reduce the surface tension of a liquid i.e. addition of detergent.
b) Temperature – rise in temperature reduces tension by weakening inter-molecular forces.
Mass and weight.
Mass is the amount of matter contained in a substance while weight is the pull of gravity on an object.
The SI unit for mass is the Kg while weight is the newton (N).
Mass is constant regardless of place while weight changes with place.
The relationship between ma ss and weight is given by the following formula, W = mg where g = gravitational force.
Differences between mass and weight Mass
- It is the quantity of matter in a body
- It is measured in kilograms
- It is the same everywhere
- It is measured using a beam balance
- Has magnitude only Weight
- It is the pull of gravity on a body
- It is measured in newton’s
- It changes from place to place
- Measured using a spring balance
- Has both magnitude and direction
2024 FORM 1 2 3 4 REVISION RESOURCES
FORM 1 2 3 4 TERM 1 2 3 OPENER , MID AND END TERM EXAMS
1995-2024 KCSE KNEC PAPERS QUESTIONS,ANSWERS AND REPORT
2008-2024 KCSE FORM 4 COUNTY MOCKS
FORM 1 2 3 4 SCHEMES OF WORK
FORM 1 2 3 4 LESSON PLANS
FORM 1 2 3 4 CLASS REVISION NOTES
FORM 1 2 3 4 TERM 1 2 3 HOLIDAY ASSISNMENTS
FORM 3 4 SETBOOKS STUDY GUIDES
FORM 1 2 3 4 TOPICAL TESTS
FORM 1 2 3 4 REVISION BOOKLETS
LIFE SKILLS NOTES
FORM 1 2 3 4 SYLLABUS
KENYA SCHOOL CODES
HOW TO REVISE AND PASS EXAMS
GUIDANCE AND CONSELLING NOTES
CLICK HERE TO DOWNLOAD ALL LATEST 2024 KCSE REVISION MOCKS
KCSE COUNTY MOCKS DOWNLOADS 2024
2023 KCSE COUNTY MOCKS DOWNLOADS
- 2023 KAPSABET BOYS POST MOCK
- PANGANI MOCK KCSE 2023
- KCSE 2023 LAINAKU II FORM 4 JOINT MOCK
- KENYA HIGH POST MOCK
- KALA MOCK =Password is- subjectcodeKALA2023
- KCSE 2023 SAMIA JOINT MOCK
LANJET 2023 EVALUATION MOCK
2023 EVALUATION MOCK nyandarua trial 4
2023 EVALUATION MOCK nyandarua trial 3
KCSE 2023 MOCKS NYARIRA CLUSTER EXAMS
KCSE 2023 CEKANA MOCKS
KCSE 2023 ACHIEVERS JOINT MOCK
- KAPSABET 2 MOCK 2023
- MOKASA 2 MOCK 2023
- 2023 Mang’u high revision mock
- FORM 4 TERM 2 BAKALE EXAM
CATHOLIC DIOCESE OF KAKAMEGA MOCK
- BSJE JOINT MOCK EXAM 2023
- MARANDA HIGH SCHOOL MOCK JUNE
- KCSE 2023 mock Nginda girls
- 2023 Kcse mock Wahundura
- 2023 Kcse mock set 22
KCSE 2023 KASSU MOCK EXAMS
- 2023 KCSE EAGLE TRIAL 1 MOCK
- 2023 lainaku revision mock
- 2023 FORM 4 evaluation exams set 18
- 2023 FORM 4 evaluation exams set 17
- 2023 FORM 4 evaluation exams set 16
LUGARI CONSTITUENCY -MOCK 1
- 2023 KCSE FORM 4 EVALUATION TEST
2023 mokasa mocks revision exams
- SUKELLEMO JOINT PRE-MOCK EXAMS
- Mumias west pre mock kcse exams
- 2023 SUNRISE PRE-MOCK
- 2023 kcse arise and shine pre-mock
- MECS CLUSTER JOINT MOCK EXAM
- Chogoria murugi zone pre-mock
- MOMALICHE 2 EXAMS PRE MOCK
- ASUMBI PRE MOCK EXAMS 2023
- 2023 MARANDA HIGH PRE-MOCK
- KAPSABET INTERNAL TRIAL 1 2023
- FORM 4 EVALUATION TEST 2023
- 2023 FORM 4 evaluation exams
Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
2022 COUNTY MOCKS 38 EXAMS
2021-22 COUNTY MOCKS 36 EXAMS
2020-21 COUNTY MOCKS 24 EXAMS
2019 COUNTY MOCKS 44 EXAMS
2018 COUNTY MOCKS 23 COUNTIES EXAMS
2017 COUNTY MOCKS 25 COUNTIES EXAMS
2016 COUNTY MOCKS 16 COUNTIES
2015 COUNTY MOCKS 20 COUNTIES
2008 , 2009 , 2010 , 2011 , 2012 , 2013, 2014 COUNTY MOCKS 25 COUNTIES
2023 KCSE COUNTY MOCKS Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
They are designed to help students get a sense of what to expect in the real exam and to identify areas where they need to improve.
The purpose of taking mock exams is to help students build confidence, develop test-taking strategies, and identify their strengths and weaknesses.
Pre-mock exams are usually taken a few weeks or months before the actual exam, while mock exams are usually taken closer to the exam date.
 WhatsApp us Now
WhatsApp us Now