Chemistry Notes Acid Bases and Indicator
Chemistry Notes Acid Bases and Indicator
Introduction To Acids, Bases And Indicator
1.In a school laboratory:
(i)An acid may be defined as a substance that tum litmus red.
(ii)A base may be defined as a substance that tum litmus blue.
Litmus is a lichen found mainly in West Africa. It changes its colour depending on Whether the solution it is in, is basic/alkaline or acidic. It is thus able to identify/show whether another substance is an acid, base or neutral.
(iii)An indicator is a substance that shows whether another substance is a base/alkaline,acid or neutral.
2.Common naturally occuring acids include:
1. Name of acid: Citric acid
Occurrence: Found in ripe citrus fruits like passion fruit/oranges/lemon
2.Name of acid:Tartaric acid
Occurrence: Found in grapes/baking powder/health salts
3.Name of acid: Lactic acid
Occurrence: Found in sour milk
4.Name of acid: Ethanoic acid
Occurrence: Found in vinegar
5.Name of acid: Methanoic acid
Occurrence: Present in ants, bees stings
6.Name of acid: Carbonic acid
Occurrence: Used in preservation of fizzy drinks like coke, Lemonade, Fanta
7.Name of acid: Butanoic acid
Occurrence: Present in cheese
8.Name of acid: Tannic acid
Occurrence: Present in tea
3.Most commonly used acids found in a school laboratory are not naturally occurring. They are manufactured. They are called mineral acids.
Common mineral acids include:
Name of mineral acid: Hydrochloric acid (HCl)
Common use: Used to clean/pickling surface of metals
:Is found in the stomach of mammals/human beings
Name of mineral acid: Sulphuric(VI) acid (HZSO4)
Common use: Used as acid in car battery, making battery, making fertilizers
Name of mineral acid: Nitric(V)acid (HNO3)
Common use: Used in making fertilizers and explosives
4.Mineral acids are manufactured to very high concentration. They are corrosive (causes painful wounds on contact with the skin) and attack/reacts With garments/clothes/metals.
In a school laboratory, they are mainly used when added a lot of Water. This is called diluting. Diluting ensures the concentration of the acid is safely low.
5. Bases are opposite of acids. Most bases do not dissolve in water.
Bases which dissolve in water are called alkalis.
Common alkalis include:
Name of alkali: Sodium hydroxide (NaOH)
Common uses: Making soaps and detergents
Name of alkali: Potassium hydroxide(KOH)
Common uses: Making soaps and detergents
Name of alkali: Ammonia solution(NH4OH)
Common uses: Making fertilizers, softening hard Water
Common bases (Which are not alkali) include:
Name of base: Magnesium oxide/hydroxide
Common name: Anti acid to treat indigestion
Name of base: Calcium oxide
Name of base: Making cement and neutralizing soil acidity
6. Indicators are useful in identifying substances which look-alike. An acid-base indicator is a substance used to identify Whether another substance is alkaline or acidic.
An acid-base indicator works by changing to different colours in neutral, acidic and alkaline solutions/dissolved in Water.
Experiment:To prepare simple acid-base indicator
Procedure
(a)Place some flowers petals in a mortar. Crush them using a pestle. Add a little sand to assist in crushing.
Add about 5cm3 of propanone/ethanol and carefully continue grinding.
Add more 5cm3 of propanone/ethanol and continue until there is enough extract in the mortar.
Filter the extract into a clean 100cm3 beaker.
(b)Place 5cm3 of filtered wood ash, soap solution, ammonia solution, sodium hydroxide, hydrochloric acid, distilled water, sulphuric(VI)acid, sour milk, sodium chloride, toothpaste and calcium hydroxide into separate test tubes.
(c)Put about three drops of the extract in (a)to each test tube in (b). Record the observations made in each case.
Sample observations
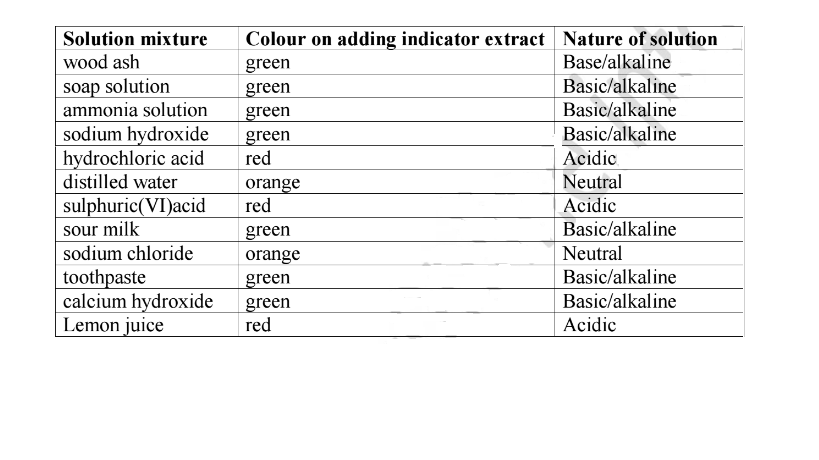
The plant extract is able to differentiate between solutions by their nature. It is changing to a similar colour for similar solutions.
(i)Since lemon juice is a known acid, then sulphuric(VI)and hydrochloric acids are similar in nature with lemon juice because the indicator show similar colours. They are acidic in nature.
(ii)Since sodium hydroxide is a known base/alkali, then the green colour of indicator shows an alkaline/basic solution.
(iii) Since pure water is neutral,then the orange colour of indicator shows neutral solutions.
7. In a school laboratory, commercial indicators are used. A commercial indicator is cheap, readily available and easy to store. Common indicators include: Litmus, phenolphthalein, methyl orange, screened methyl orange, bromothymol blue.
2024 FORM 1 2 3 4 REVISION RESOURCES
FORM 1 2 3 4 TERM 1 2 3 OPENER , MID AND END TERM EXAMS
1995-2024 KCSE KNEC PAPERS QUESTIONS,ANSWERS AND REPORT
2008-2024 KCSE FORM 4 COUNTY MOCKS
FORM 1 2 3 4 SCHEMES OF WORK
FORM 1 2 3 4 LESSON PLANS
FORM 1 2 3 4 CLASS REVISION NOTES
FORM 1 2 3 4 TERM 1 2 3 HOLIDAY ASSISNMENTS
FORM 3 4 SETBOOKS STUDY GUIDES
FORM 1 2 3 4 TOPICAL TESTS
FORM 1 2 3 4 REVISION BOOKLETS
LIFE SKILLS NOTES
FORM 1 2 3 4 SYLLABUS
KENYA SCHOOL CODES
HOW TO REVISE AND PASS EXAMS
GUIDANCE AND CONSELLING NOTES
CLICK HERE TO DOWNLOAD ALL LATEST 2024 KCSE REVISION MOCKS
KCSE COUNTY MOCKS DOWNLOADS 2024
2023 KCSE COUNTY MOCKS DOWNLOADS
- 2023 KAPSABET BOYS POST MOCK
- PANGANI MOCK KCSE 2023
- KCSE 2023 LAINAKU II FORM 4 JOINT MOCK
- KENYA HIGH POST MOCK
- KALA MOCK =Password is- subjectcodeKALA2023
- KCSE 2023 SAMIA JOINT MOCK
LANJET 2023 EVALUATION MOCK
2023 EVALUATION MOCK nyandarua trial 4
2023 EVALUATION MOCK nyandarua trial 3
KCSE 2023 MOCKS NYARIRA CLUSTER EXAMS
KCSE 2023 CEKANA MOCKS
KCSE 2023 ACHIEVERS JOINT MOCK
- KAPSABET 2 MOCK 2023
- MOKASA 2 MOCK 2023
- 2023 Mang’u high revision mock
- FORM 4 TERM 2 BAKALE EXAM
CATHOLIC DIOCESE OF KAKAMEGA MOCK
- BSJE JOINT MOCK EXAM 2023
- MARANDA HIGH SCHOOL MOCK JUNE
- KCSE 2023 mock Nginda girls
- 2023 Kcse mock Wahundura
- 2023 Kcse mock set 22
KCSE 2023 KASSU MOCK EXAMS
- 2023 KCSE EAGLE TRIAL 1 MOCK
- 2023 lainaku revision mock
- 2023 FORM 4 evaluation exams set 18
- 2023 FORM 4 evaluation exams set 17
- 2023 FORM 4 evaluation exams set 16
LUGARI CONSTITUENCY -MOCK 1
- 2023 KCSE FORM 4 EVALUATION TEST
2023 mokasa mocks revision exams
- SUKELLEMO JOINT PRE-MOCK EXAMS
- Mumias west pre mock kcse exams
- 2023 SUNRISE PRE-MOCK
- 2023 kcse arise and shine pre-mock
- MECS CLUSTER JOINT MOCK EXAM
- Chogoria murugi zone pre-mock
- MOMALICHE 2 EXAMS PRE MOCK
- ASUMBI PRE MOCK EXAMS 2023
- 2023 MARANDA HIGH PRE-MOCK
- KAPSABET INTERNAL TRIAL 1 2023
- FORM 4 EVALUATION TEST 2023
- 2023 FORM 4 evaluation exams
Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
2022 COUNTY MOCKS 38 EXAMS
2021-22 COUNTY MOCKS 36 EXAMS
2020-21 COUNTY MOCKS 24 EXAMS
2019 COUNTY MOCKS 44 EXAMS
2018 COUNTY MOCKS 23 COUNTIES EXAMS
2017 COUNTY MOCKS 25 COUNTIES EXAMS
2016 COUNTY MOCKS 16 COUNTIES
2015 COUNTY MOCKS 20 COUNTIES
2008 , 2009 , 2010 , 2011 , 2012 , 2013, 2014 COUNTY MOCKS 25 COUNTIES
2023 KCSE COUNTY MOCKS Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
They are designed to help students get a sense of what to expect in the real exam and to identify areas where they need to improve.
The purpose of taking mock exams is to help students build confidence, develop test-taking strategies, and identify their strengths and weaknesses.
Pre-mock exams are usually taken a few weeks or months before the actual exam, while mock exams are usually taken closer to the exam date.
 WhatsApp us Now
WhatsApp us Now