Chemistry Notes Acid Bases and Salts
Chemistry Notes Acid Bases and Salts
K.C.S.E Online Revision
A. Acids And Bases
At a school laboratory:
(i)An acid may be defined as a substance that turn litmus red.
(ii)A base may be defined as a substance that turn litmus blue.
Litmus is a lichen found mainly in West Africa. It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.It is thus able to identify/ show whether
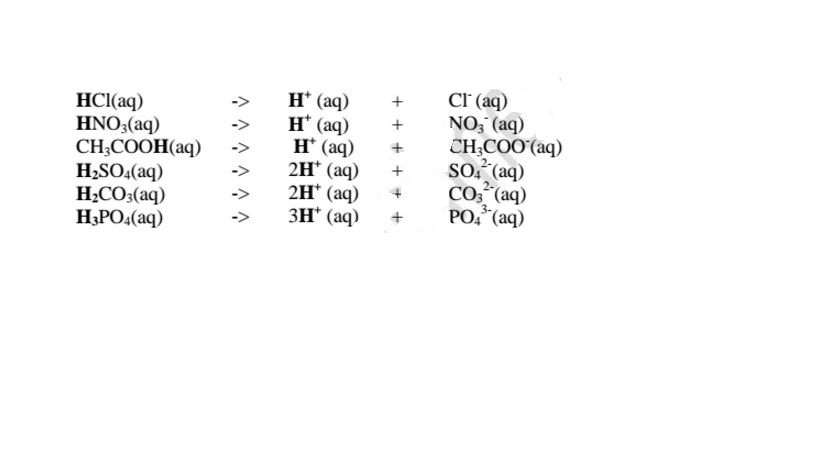
1. An acid is a substance that dissolves in water to form H+/H3O+ as the only positive ion/cation. This is called the Arrhenius definition of an acid. From this definition, an acid dissociate/ionize in water releasing H+ thus:
2.A base is a substance which dissolves in water to form OH‘ as the only negatively charged ion/anion.
This is called Arrhenius definition of a base.
From this definition, a base dissociate/ionize in Water releasing OH’ thus:

3. An acid is a proton donor.
A base is a proton acceptor.
This is called Bronsted-Lowry definition of acids and bases.
From this definition, an acid donates H+ .
H+ has no electrons and neutrons .It contains only a proton.
Examples
i. From the equation:
(a)(i)For the forward reaction from left to right, H2O gains a proton to form H3O+ and thus H2o is a proton acceptor.It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, H3O+ donates a proton to form H2o and thus H3O+ is an ,,opposite” proton donor. It is a Bronsted- Lowry conjugate acid
(b)(i)For the forward reaction from left to right, HCl donates a proton to form Cl‘ and thus HCl is a proton donor.
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl” gains a proton to form HCl and thus Cl’ is an ,,opposite” proton acceptor.
It is a Bronsted-Lowry conjugate base.
Every base /acid from Bronsted-Lowry definition thus must have a conjugate product/reactant.
II. From the equation:
(a)(i)For the forward reaction from left to right, NH; gains a proton to form NH4 and thus NH3; is a proton acceptor .
It is a Bronsted-Lowry base
(ii) For the backward reaction from right to left, NH4+ donates a proton to form NH; and thus NH4+ is an ,,opposite” proton donor.
It is a Bronsted-Lowry conjugate acid
(b)(i)F or the forward reaction from left to right, HCl donates a proton to form C1‘ and thus HCl is a proton donor .
It is a Bronsted-Lowry acid
(ii) For the backward reaction from right to left, Cl” gains a proton to form HCl and thus Cl’ is an ,,opposite” proton acceptor.
It is a Bronsted-Lowry conjugate base.
4. Acids and bases show acidic and alkaline properties/characteristics only in Water but not in other solvents e.g.
(a)Hydrogen chloride gas dissolves in water to form hydrochloric acid
Hydrochloric acid dissociates/ionizes in water to free
ions. The free
ions are responsible for:
(i)turning blue litmus paper/solution red.
(ii)show pH value 1/2/3/4/5/6
(iii)are good electrolytes/conductors of electricity/undergo electrolysis.
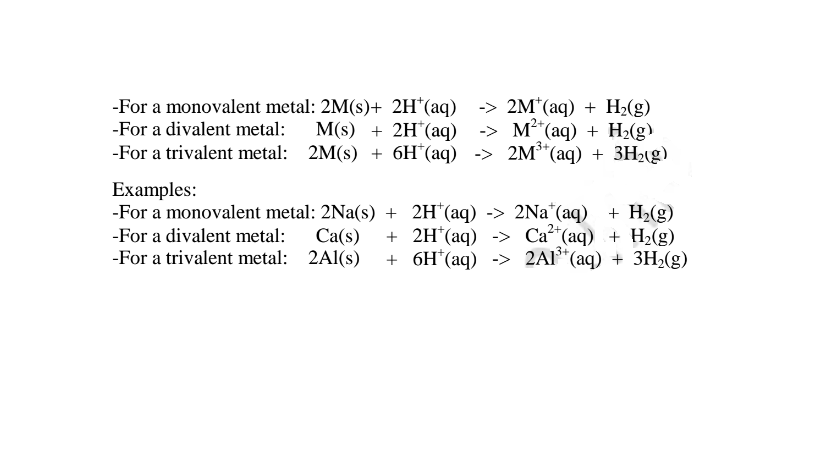
(iv)react with metals to produce /evolve hydrogen gas and a salt. i.e.
Ionically:
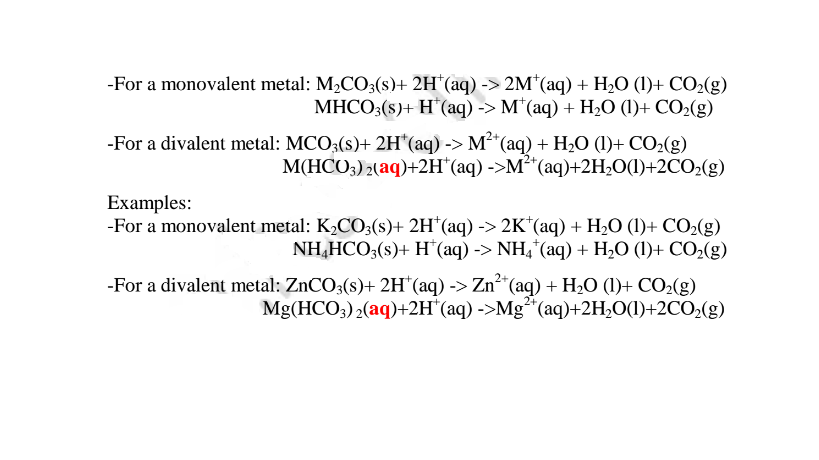
(v) react with metal carbonates and hydrogen carbonates to produce /evolve carbon(IV)oxide gas ,Water and a salt. i.e.
lonically:
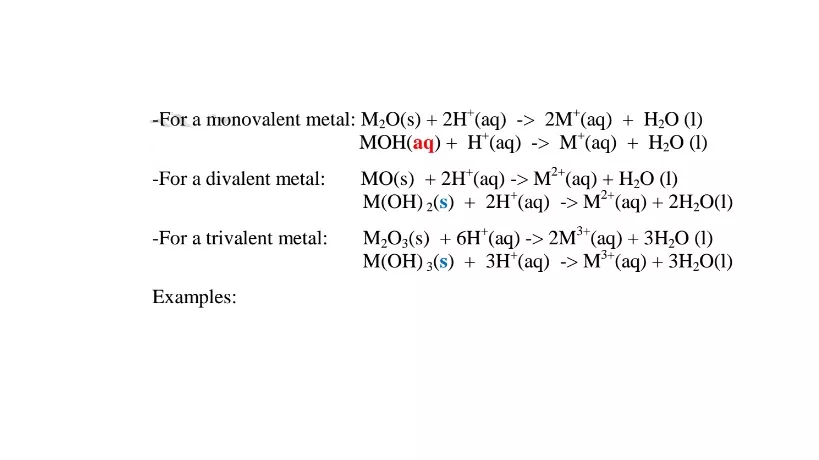
(vi)neutralize metal oxides/hydroxides to salt and water only. i.e. Ionically:
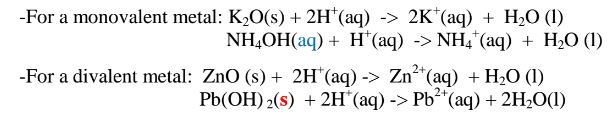
(b)Hydrogen chloride gas dissolves in methylbenzene /benzene but does not dissociate /ionize into free ions.
It exists in molecular state showing none of the above properties.
(c)Ammonia gas dissolves in water to form aqueous ammonia which dissociate/ionize to free NH4+ (aq) and OH'(aq) ions.
This dissociation/ionization makes aqueous ammonia to:
(i)tum litmus paper/solution blue.
(ii)have pH 8/9/ 10/ 11
(iii)be a good electrical conductor
(iv)react with acids to form ammonium salt and water only.
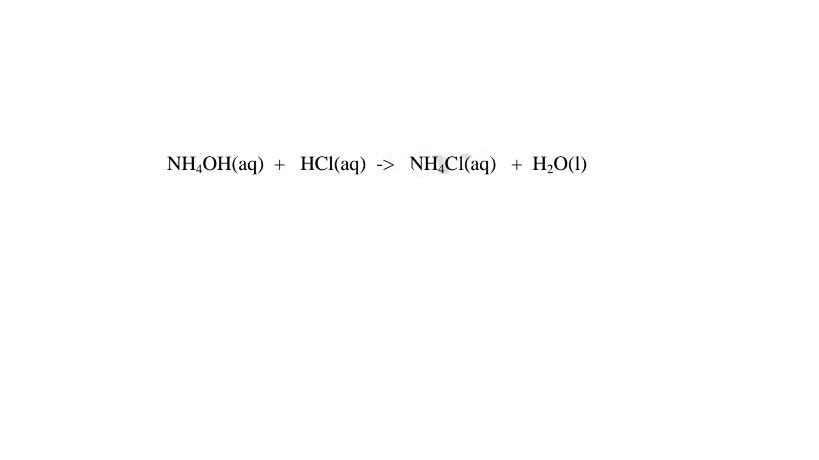
(d)Ammonia gas dissolves in methylbenzene/benzene /kerosene but does not dissociate into free ions therefore existing as molecules
6. Solvents are either polar or non-polar.
A polar solvent is one which dissolves ionic compounds and other polar solvents.
Water is polar solvent that dissolves ionic and polar substance by surrounding the free ions as below:
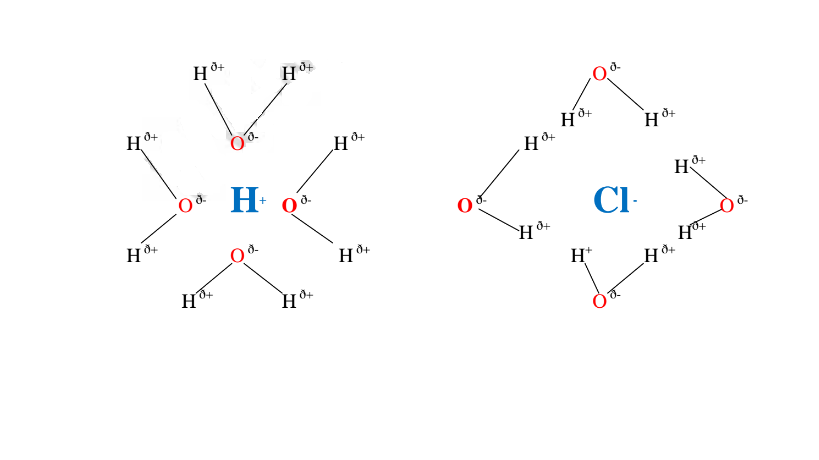
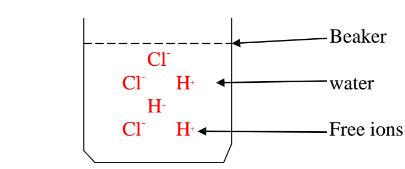
Note:Water is polar .It is made up of :
Oxygen atom is partially negative and two hydrogen atoms which are partially positive.
They surround the free Hl and Cl” ions.
A non polar solvent is one which dissolved non-polar substances and covalent
compounds.
If a polar ionic compound is dissolved in non-polar solvent ,it does not ionize/dissociate into free ions as below:
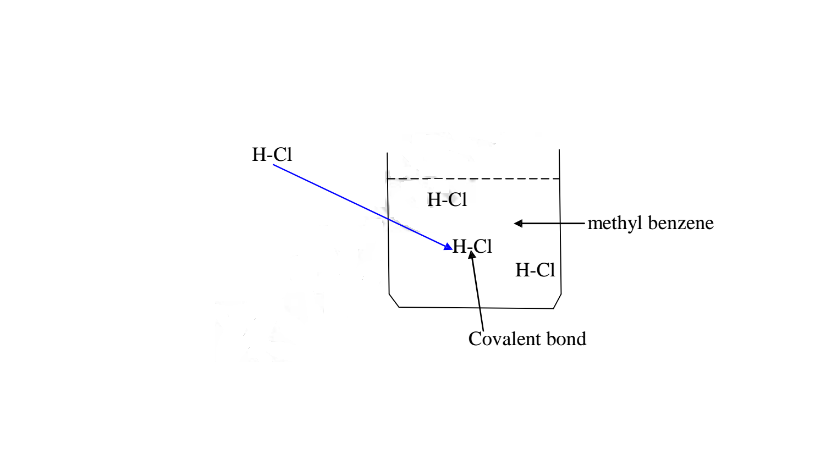
7. Some acids and bases are strong while others are Weak.
(a)A strong acid/base is one which is fully/wholly/completely dissociated / ionized into many free H+ /OH’ ions i.e.
i. Strong acids exists more as free H+ ions than molecules. e. g.
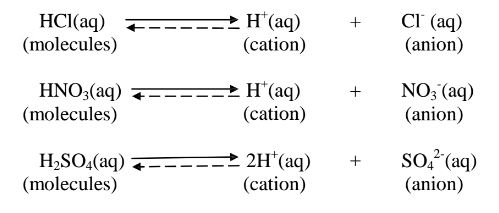
ii. Strong bases/alkalis exists more as free OH’ ions than molecules. e.g.
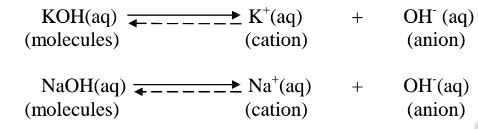
(b) A weak base/acid is one which is paitially /partly dissociated /ionized in water into free OH’ (aq) and H+(aq) ions.
i. Weak acids exists more as molecules than as free Hl ions. e.g.
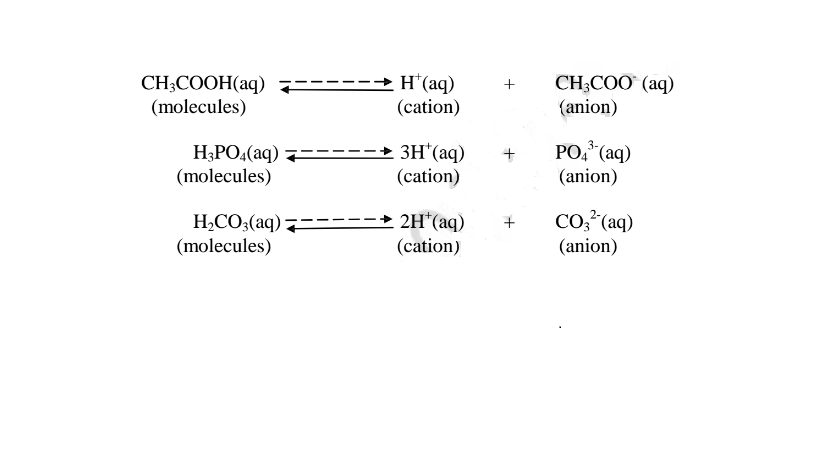
ii. Weak bases/alkalis exists more as molecules than free OH’ ions. e. g.
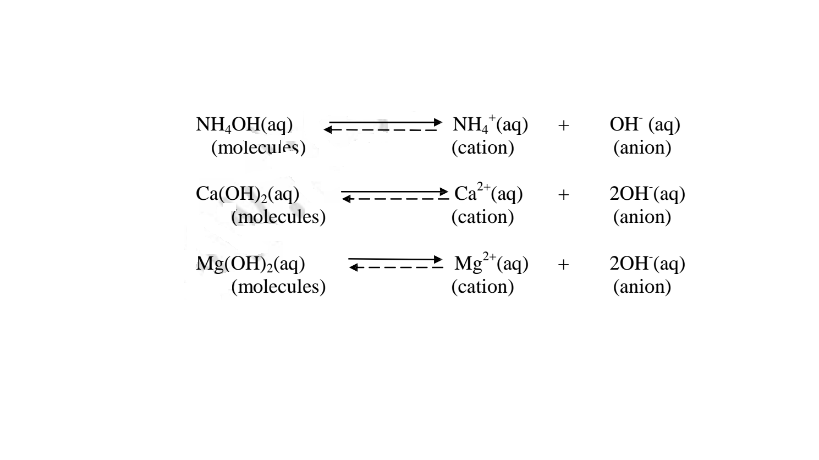
8. The concentration of an acid/base/alkali is based on the number of moles of acid/bases dissolved in a decimeter(litre)of the solution.
2024 FORM 1 2 3 4 REVISION RESOURCES
FORM 1 2 3 4 TERM 1 2 3 OPENER , MID AND END TERM EXAMS
1995-2024 KCSE KNEC PAPERS QUESTIONS,ANSWERS AND REPORT
2008-2024 KCSE FORM 4 COUNTY MOCKS
FORM 1 2 3 4 SCHEMES OF WORK
FORM 1 2 3 4 LESSON PLANS
FORM 1 2 3 4 CLASS REVISION NOTES
FORM 1 2 3 4 TERM 1 2 3 HOLIDAY ASSISNMENTS
FORM 3 4 SETBOOKS STUDY GUIDES
FORM 1 2 3 4 TOPICAL TESTS
FORM 1 2 3 4 REVISION BOOKLETS
LIFE SKILLS NOTES
FORM 1 2 3 4 SYLLABUS
KENYA SCHOOL CODES
HOW TO REVISE AND PASS EXAMS
GUIDANCE AND CONSELLING NOTES
CLICK HERE TO DOWNLOAD ALL LATEST 2024 KCSE REVISION MOCKS
KCSE COUNTY MOCKS DOWNLOADS 2024
2023 KCSE COUNTY MOCKS DOWNLOADS
- 2023 KAPSABET BOYS POST MOCK
- PANGANI MOCK KCSE 2023
- KCSE 2023 LAINAKU II FORM 4 JOINT MOCK
- KENYA HIGH POST MOCK
- KALA MOCK =Password is- subjectcodeKALA2023
- KCSE 2023 SAMIA JOINT MOCK
LANJET 2023 EVALUATION MOCK
2023 EVALUATION MOCK nyandarua trial 4
2023 EVALUATION MOCK nyandarua trial 3
KCSE 2023 MOCKS NYARIRA CLUSTER EXAMS
KCSE 2023 CEKANA MOCKS
KCSE 2023 ACHIEVERS JOINT MOCK
- KAPSABET 2 MOCK 2023
- MOKASA 2 MOCK 2023
- 2023 Mang’u high revision mock
- FORM 4 TERM 2 BAKALE EXAM
CATHOLIC DIOCESE OF KAKAMEGA MOCK
- BSJE JOINT MOCK EXAM 2023
- MARANDA HIGH SCHOOL MOCK JUNE
- KCSE 2023 mock Nginda girls
- 2023 Kcse mock Wahundura
- 2023 Kcse mock set 22
KCSE 2023 KASSU MOCK EXAMS
- 2023 KCSE EAGLE TRIAL 1 MOCK
- 2023 lainaku revision mock
- 2023 FORM 4 evaluation exams set 18
- 2023 FORM 4 evaluation exams set 17
- 2023 FORM 4 evaluation exams set 16
LUGARI CONSTITUENCY -MOCK 1
- 2023 KCSE FORM 4 EVALUATION TEST
2023 mokasa mocks revision exams
- SUKELLEMO JOINT PRE-MOCK EXAMS
- Mumias west pre mock kcse exams
- 2023 SUNRISE PRE-MOCK
- 2023 kcse arise and shine pre-mock
- MECS CLUSTER JOINT MOCK EXAM
- Chogoria murugi zone pre-mock
- MOMALICHE 2 EXAMS PRE MOCK
- ASUMBI PRE MOCK EXAMS 2023
- 2023 MARANDA HIGH PRE-MOCK
- KAPSABET INTERNAL TRIAL 1 2023
- FORM 4 EVALUATION TEST 2023
- 2023 FORM 4 evaluation exams
Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
2022 COUNTY MOCKS 38 EXAMS
2021-22 COUNTY MOCKS 36 EXAMS
2020-21 COUNTY MOCKS 24 EXAMS
2019 COUNTY MOCKS 44 EXAMS
2018 COUNTY MOCKS 23 COUNTIES EXAMS
2017 COUNTY MOCKS 25 COUNTIES EXAMS
2016 COUNTY MOCKS 16 COUNTIES
2015 COUNTY MOCKS 20 COUNTIES
2008 , 2009 , 2010 , 2011 , 2012 , 2013, 2014 COUNTY MOCKS 25 COUNTIES
2023 KCSE COUNTY MOCKS Mock exams and pre-mock exams are practice tests that are taken before the actual exams.
They are designed to help students get a sense of what to expect in the real exam and to identify areas where they need to improve.
The purpose of taking mock exams is to help students build confidence, develop test-taking strategies, and identify their strengths and weaknesses.
Pre-mock exams are usually taken a few weeks or months before the actual exam, while mock exams are usually taken closer to the exam date.
 WhatsApp us Now
WhatsApp us Now