Chemistry Form 1 Topical Revision
Chemistry Form 1 Topical Revision
Questions
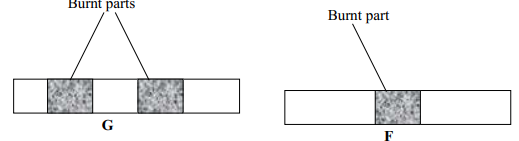
- Wooden splints F and G were placed in different zones of a Bunsen burner flame.
The diagram below gives the observations that were made
- Explain the difference between F and G
- Name the type of flame that was used in the above experiment
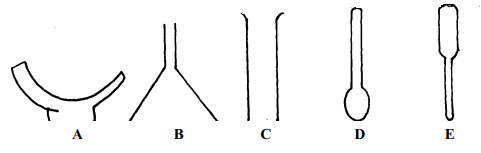
- The diagrams below represent a list of apparatus which are commonly used in a chemistry laboratory:-

- Give the correct order of the apparatus, using the letters only, to show the correct arrangement that can be used to prepare and investigate the nature of PH of a sample of onion solution
- Name one chemical substance and apparatus that is needed in this experiment
-
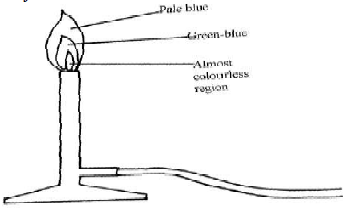
- When the air-hole is fully opened, the bunsen burner produces a non-luminous flame. Explain
- Draw a labelled diagram of a non-luminous flame
- What is a drug?
- Give two drugs that are commonly abused by the youth.
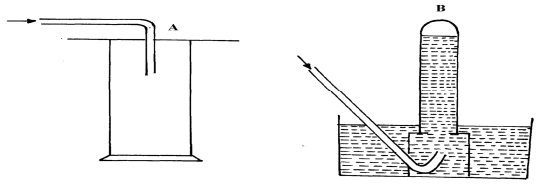
- The diagram below shows three methods for collecting gases in the laboratory
- Name the methods A and B
- From the methods above, identify one that is suitable for collecting sulphur (IV) oxide. Explain
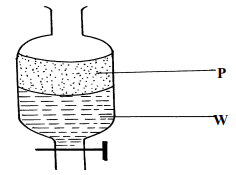
- A mixture of hexane and water was shaken and left to separate as shown in the diagram below:
State the identity of;
(i) P ………………………………..…….. (ii) W ………………………………….…. - The diagrams below are some common laboratory apparatus. Name each apparatus and state its use
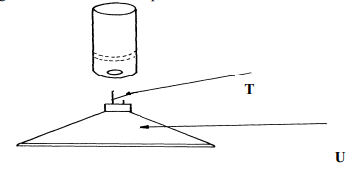
Diagram Name Use (½mk ) (½mk) (½mk) (½mk) - The diagram below shows some parts of a Bunsen burner
Explain how the parts labelled T and U are suited to their functions
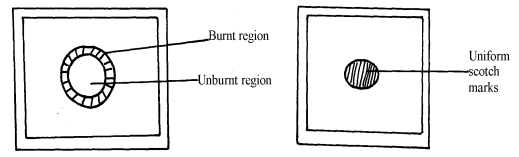
- The diagram below shows the appearance of two pieces of paper placed in different parts of a non-luminous flame of a Bunsen burner and removed quickly before they caught fire.
- What do the experiments show about the outer region of the flame?
- From the above experiment, which part of the flame is better to use for heating? Give a reason
- A crystal of copper (II) sulphate was placed in a beaker of water. The beaker was left standing for two days without shaking. State and explain the observations that were made.
- Study the information in the table below and answer questions that follow. (Letters given are not real symbols)
Ions Electron arrangement Ionic radius (nm) A+
B+
C2+2.8
2.8.8
2.80.95
0.133
0.065Explain why the ionic radius of :-
- B+ is greater than that of A+
- C2+ is smaller than the of A+
Answers
- F is place in the middle of the flame while G is placed at the upper region of the flame
- Non – luminous flame
- The laboratory gas burns in excess oxygen
OR burns completely or produces CO2 and H2O only
– No unburnt carbon remains
OR No soot is formed/Produced.
- The laboratory gas burns in excess oxygen
- a substance which when taken alters the body chemistry
- – alcohol
– Tobacco
- A- Downward delivery /upward displacement of air
B – Over water - A – Denser than air
- A- Downward delivery /upward displacement of air
- P – Haxane
- W – Water
- Name – Mortar. √½
Use – Holding solid substances being crushed. √½
Name – Crucible √½
Use – Holding solid elements being heated strongly. √½ - T – has a very small hole which releases the gas in small quantities/in form of a jet.
U – It is heavy for stability - It is very hot. (1 mk) √1
- The upper√1 part. Because all the gases undergo complete √1 combustion. √1 (2 mks) 3
- The crystal dissolved√ ½ . Blue color spreads in water √ ½ . The crystal broke up into smaller particles of copper (II) sulphate and diffused in all direction
- W has more energy levels than S. √1
- C has got (12) protons pulling the 10 electrons while A has 11 protons
2 pulling 10 electrons. √1

 WhatsApp us Now
WhatsApp us Now